Welcome to Our Company
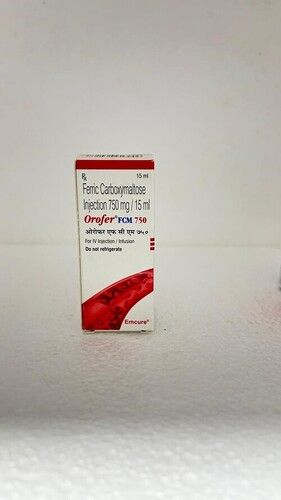
Orofer Fcm 750 mg injection
2500 INR/Piece
Product Details:
- Appearance Dark brown clear liquid
- Assay Ferric Carboxymaltose 750 mg/vial
- Solubility Soluble in water
- Melting Point Not applicable for liquid pharmaceutical formulations
- CAS No 9007-72-1
- Molecular Formula C6H16Fe2O15
- HS Code 30049099
- Click to View more
X
Orofer Fcm 750 mg injection Price And Quantity
- 2500 INR/Piece
- 5 Piece
Orofer Fcm 750 mg injection Product Specifications
- High pharmaceutical-grade purity
- 30049099
- C6H16Fe2O15
- Pharmaceutical Injection
- 24 months
- 9007-72-1
- Ferric Carboxymaltose (750 mg)
- Soluble in water
- IV iron replacement therapy for treatment of iron deficiency anemia
- Store below 25C protect from light and moisture. Do not freeze.
- Not applicable for liquid pharmaceutical formulations
- Ferric Carboxymaltose 750 mg/vial
- Dark brown clear liquid
Product Description
Orofer Fcm 750 mg injection is a high pharmaceutical-grade purity IV iron replacement therapy for the treatment of iron deficiency anemia. It contains Ferric Carboxymaltose (750 mg) as the active ingredient. The dark brown clear liquid is soluble in water and is stored below 25C to protect from light and moisture. With a shelf life of 24 months, this pharmaceutical injection is ideal for those in need of iron supplementation.
FAQs of Orofer Fcm 750 mg injection:
Q: What is the active ingredient in Orofer Fcm 750 mg injection?
A: The active ingredient in Orofer Fcm 750 mg injection is Ferric Carboxymaltose (750 mg).Q: How should Orofer Fcm 750 mg injection be stored?
A: Orofer Fcm 750 mg injection should be stored below 25C, protected from light and moisture. It should not be frozen.Q: What is the shelf life of Orofer Fcm 750 mg injection?
A: The shelf life of Orofer Fcm 750 mg injection is 24 months.Q: Is Orofer Fcm 750 mg injection suitable for IV iron replacement therapy?
A: Yes, Orofer Fcm 750 mg injection is specifically designed for IV iron replacement therapy for the treatment of iron deficiency anemia.Q: What is the appearance of Orofer Fcm 750 mg injection?
A: Orofer Fcm 750 mg injection appears as a dark brown clear liquid.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email





 Call Me Free
Call Me Free
